Opportunities like this one don’t come around very often. Here, Autocar has the chance to build its own lithium ion electric car battery cell from scratch, to see the inner workings of a device that usually stays closed because its contents are flammable and to hear an expert ‘how it works’ explanation from the UK’s foremost professor of EV batteries.
There are only a couple of places in the UK where batteries are built from raw materials and one of them is the prototype manufacturing plant at Warwick Manufacturing Group (WMG), a department of the illustrious University of Warwick. Our mentor for the day is Professor David Greenwood, a brilliant communicator of the intricate flows of electrons and lithium ions inside these anonymous-looking grey packages. So free your mind of the notion of crankshafts, valves and bearings and introduce to it instead the anodes, cathodes, separators and LiPF6 electrolytes of our electrically driven future.
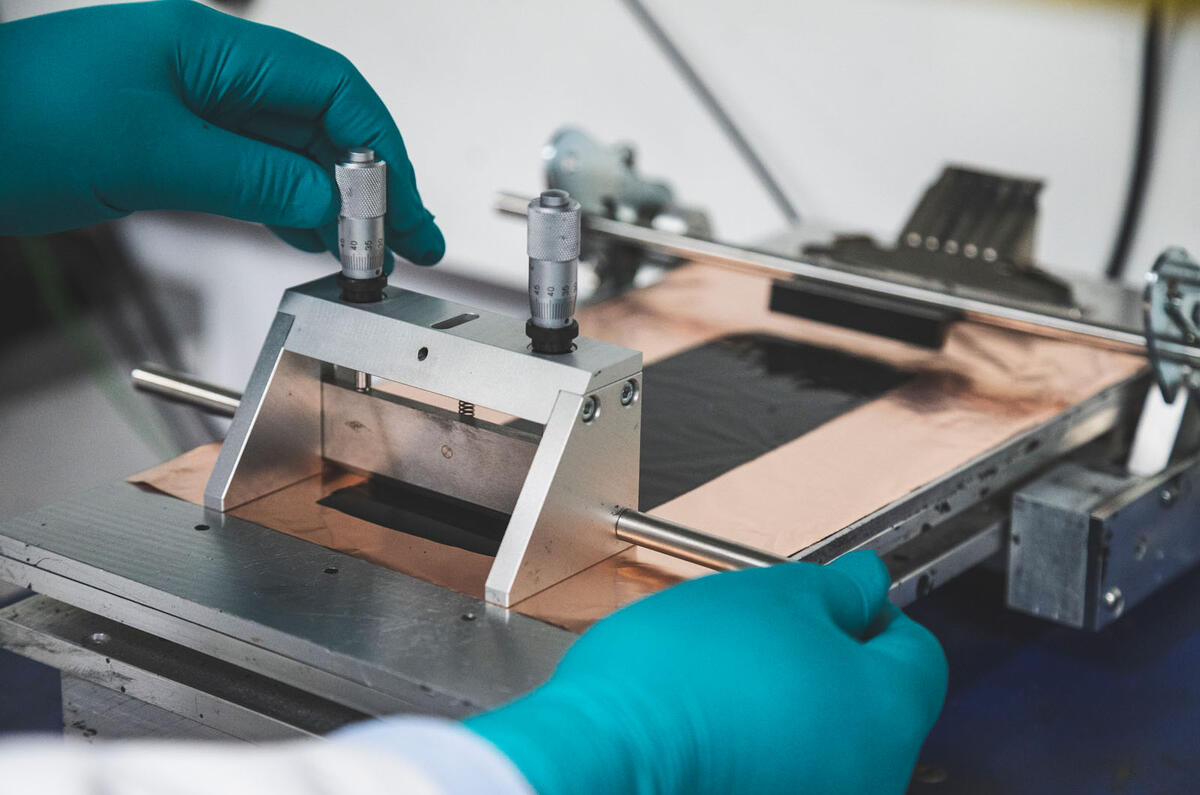
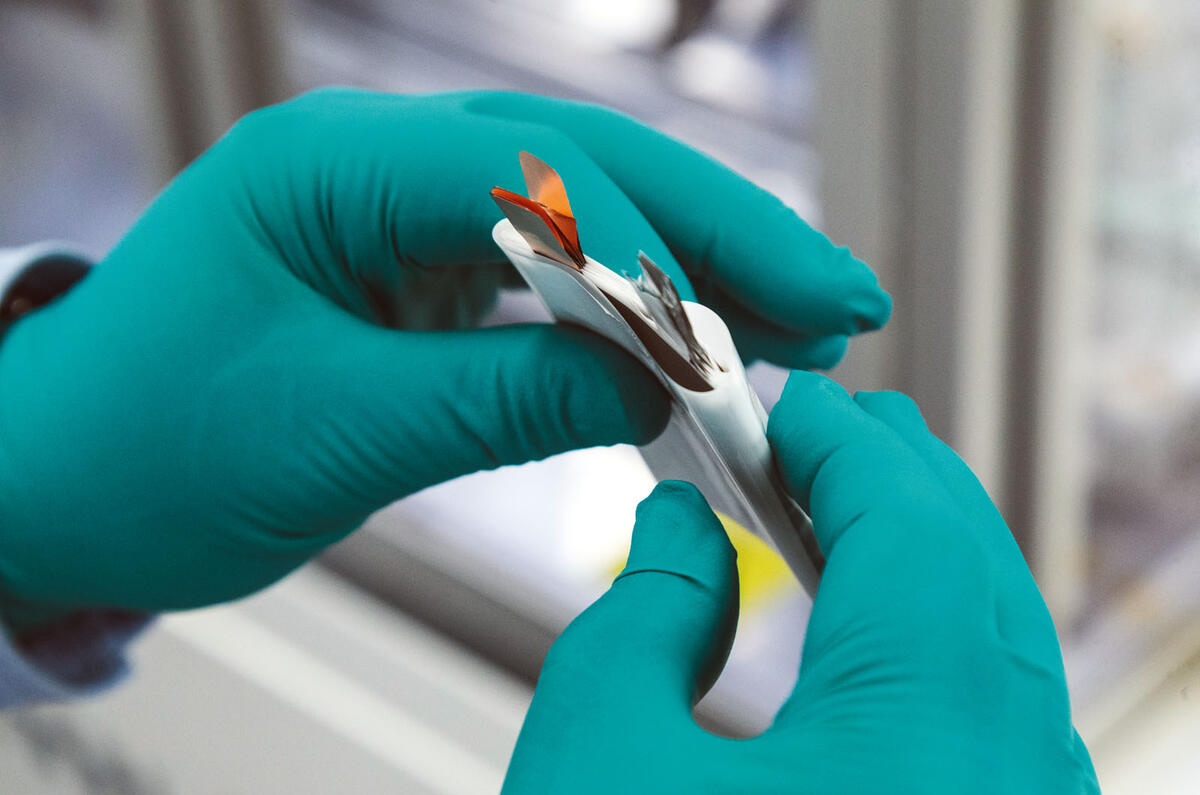
Our task is to make a small 3.5V A7-sized pouch cell – about the size of a credit card at 50mm by 70mm – using machinery on WMG’s prototype production line. In a production car, like the Nissan Leaf or Jaguar I-Pace, dozens of these pouches are built up into modules before the modules are wired together to make the battery.
The internal parts and the principles of design and build are the same as for bigger pouch cells, like those fitted to the Leaf, but also the prismatic designs and cylinders used by Tesla. Tesla’s 18650 cells may be a very different shape but they contain the same basic componentry, just rolled up inside a tube.
There are four main processes – make the anode, make the cathode, pack them into a pouch and fill with the liquid electrolyte that contains the vital lithium – but in total there are 11 manufacturing steps. Our session will take a couple of hours, but in a volume production plant, like Tesla’s Gigafactory, 20 cells a second roll off the production lines. Time to don the protective green rubber gloves, white lab coat and go mix chemicals.

Making the Autocar battery
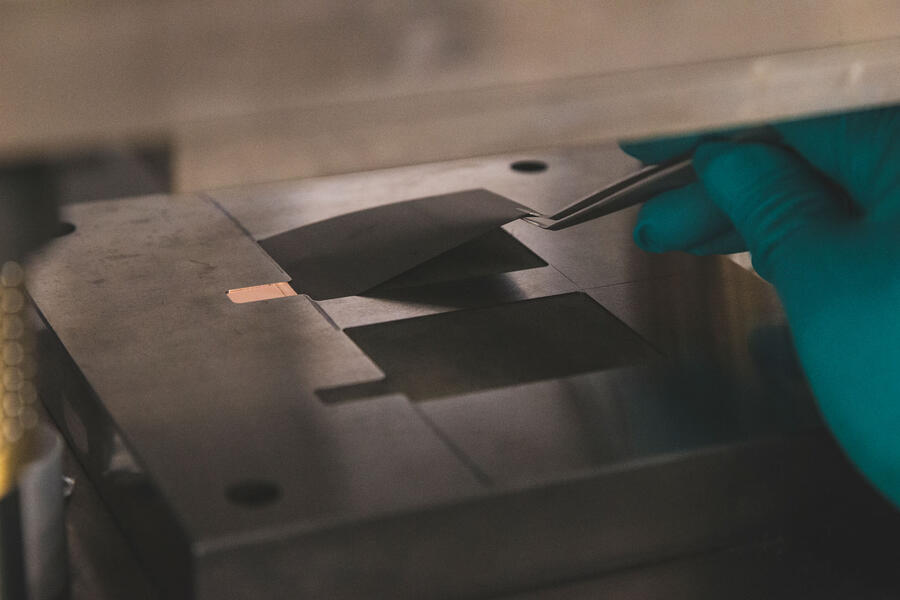
1: MATERIAL WEIGHING FOR ANODE
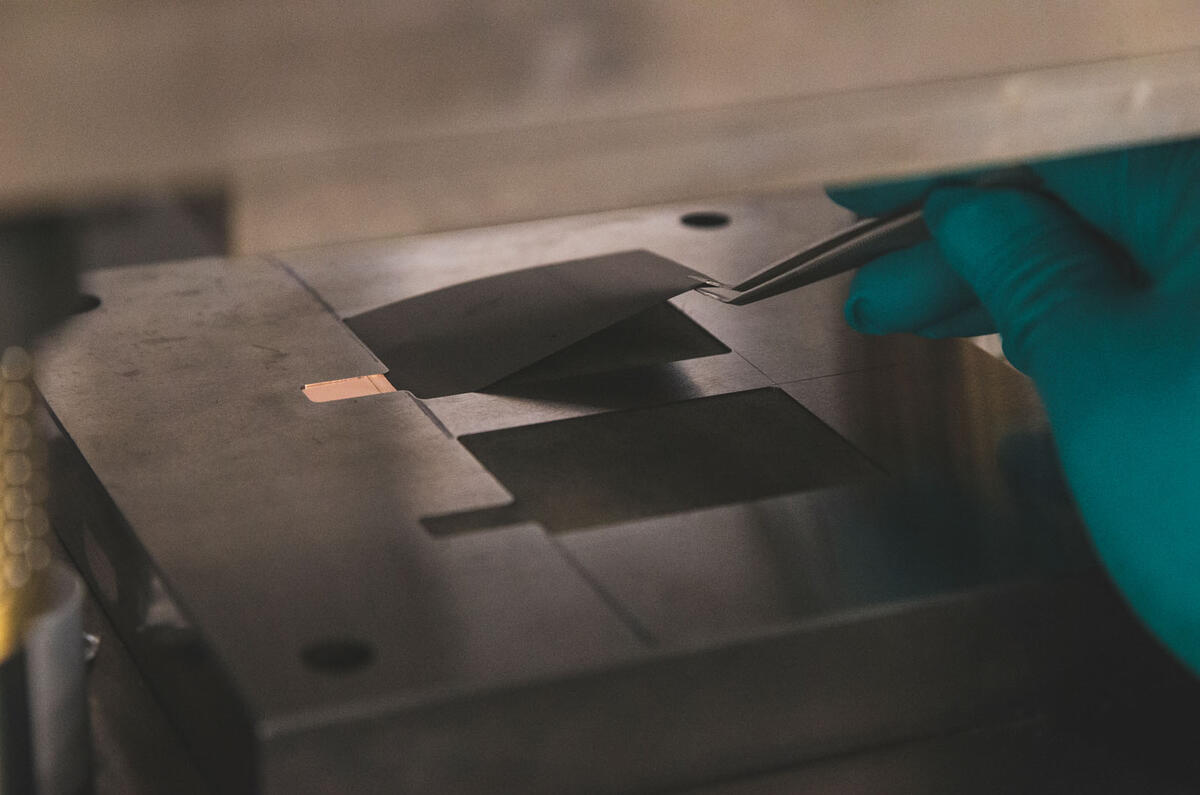
The electrochemically active ingredient in the anode is graphite – it’s the component that actually stores lithium ions. It comes as a fine powder, each particle of which is just 10 microns across – one fifth of a human hair. Here, the graphite is mixed in a water-based solvent with carbon black and a latex-based binder to make a slurry.
The carbon makes the graphite electrically conductive and the binder is an adhesive so it will stick to a copper backing plate in the next stage. A very similar process is used to make the cathode, but the very fine nickel particles can only be handled while wearing pressurised breathing masks, so aren’t appropriate for our visit.














































Join the debate
Add your comment
Not built from scratch
Would have been more interesting if you'd gone and mined the lithium and cobalt alongside the seven year old kids who have to do it every day.
Whereabouts
You have no idea where the lithium came from or who mined it in this experiment!
You mean like in your phone?...
Artisanal mining makes up only a small fragment of the Cobalt market in the DRC, and has nothing to do with Lithium. There is more of this kind of Cobalt in phones and laptops produced for decades than car because of the clear CSR issues.. please educate yourself.
As for effect on kids, I would say the effect on kids on the Niger Delta in Nigeria due to oil spills and contamination of marine life is much more of an issue, but no one talks about this due they because oil is good and batteries are bad for the planet.
Yes, an excellent article
The article was really interesting to read. I'd love to hear more about other technical details, for both electric and internal combustion cars.
Excellent Article
Please do more of these type of articles.